Insights
Insights

NICE refines the HST routing criteria: What you need to know
Written by Brian O'Toole The National Institute for Health and Care Excellence (NICE) Highly Specialised Technologies (HST) programme is designed to evaluate innovative treatments for rare and complex conditions. On 19th March ... Read more

Systematic review case study: scoping research to identify comparators
Written by Sneha Bhadti and David Pritchett Establishing a list of relevant comparators at the protocol development stage is a vital step in the systematic literature review (SLR) process. Scoping research during this stage can help ... Read more

National Numeracy Day 2025: Why money and maths matter in health economics
Written by Abby Paine and Dom Partridge This is our third year celebrating National Numeracy Day, and this year’s theme of money is especially relevant for those of us working in health economics and market access. In previous years, ... Read more

Maximising SLRs for HEOR – why PICO matters
Written by Susan O’Connell and Sara Bartlome Introduction Systematic literature reviews (SLRs) seek to answer a specific research question by translating the question into search terms and developing a literature search strategy to ... Read more

A maths career in pharma all adds up, says Source Health Economics
Written by Abby Paine and Jack Scerri What better way to spend a Saturday morning than volunteering to lead an interactive mathematics workshop for a group of enthusiastic young students! That’s exactly what two of our staff did ... Read more

One year in HEOR: growing as medical writers at Source
Written by Kelly O’Toole and Ethan MaughanMedical writers working in health economics and outcome research (HEOR) play a crucial role in helping to communicate clinical and economic data in a clear and accurate way. At Source, medical writers ... Read more

Rare Disease Day 2025 – Challenges in data gathering
Written by Aurore Sommer, Medical Writer A disease is considered to be rare when it affects fewer than 1 in 2,000 individuals. To this day, more than 6,000 rare diseases have been identified, affecting around 300 million people ... Read more

World Cancer Day 2025
Today is World Cancer Day! The current campaign is ‘United by Unique’, which highlights the importance of personalised care to cater to each patient’s unique needs. In honour of World Cancer Day, Source has taken a look back at our 45 ... Read more

Manuscript deep dive
At Source, we pride ourselves on our tailored, strategic, and comprehensive approach to supporting our clients with their publications. Please click through the carousel below for more information, and please get in touch to discover how Source ... Read more

Sustainability and the future of HTAs
Exploring sustainability, climate change, and how including environmental data in HTAs could help healthcare reach sustainability goals Read more

World AIDS day 2024
Written by Emma Lones, Senior Medical Writer This year, the campaign is focused on how individuals can help to end HIV stigma and make the world a better place for people living with HIV. In honour of World AIDS Day, Source has ... Read more

Factual accuracy checking
Written by Emma Lones, Senior Medical Writer Health technology assessments (HTA) include a vast amount of data and technical information. We regularly check the factual accuracy of documents written by HTA assessors during the ... Read more

The power of storytelling in medical communications
SCIRIS brings together five outstanding agencies with a team of over 300 individuals who are driven to deliver excellence in communications and consulting. Our first SCIRIS guest blog is on the power of storytelling in medical communications ... Read more

Language restrictions in systematic literature reviews: a reflection on the current tone
Written by Alys Ridsdale, Associate Systematic Review Analyst Introduction There are over 7,100 spoken languages across the globe (1). Despite this linguistic diversity, including language restrictions in a systematic literature ... Read more

What are the implications of joint clinical assessment (JCA) on systematic reviews?
This article offers a overview of the challenges and strategies for navigating the upcoming EU JCA process, focusing on managing PICOS criteria and deadlines in clinical systematic literature reviews (SLRs). Read more

The European Union Joint Clinical Assessment (EU JCA) – an overview
This article offers a brief overview of the EU JCA process and is the first in a series of articles where we will discuss the new process and implications for HTA developers. Read more

Pros and cons of crowdsourcing for systematic review
Written by Ciara Thomas, Vicky Crowe, & David Pritchett Introduction To achieve optimal patient outcomes, systematic literature reviews (SLRs) must synthesise high quality, contemporary evidence to inform health policy and ... Read more

National Numeracy Day 2024: making maths work for women and girls
Written by Abby Paine, Hollie Pilkington, and Dom Partridge Two years ago we wrote a blog for National Numeracy Day 2022, and enjoyed the process so much that we thought we would write another one! Last time, we wrote about network ... Read more

Quality assessment in single-arm trials: insights for systematic reviews
Written by Hannah Shapiro Quality assessment in single-arm trials Quality assessment is a vital aspect of conducting a thorough systematic literature review (SLR), as the validity of the conclusions of the review depends on the ... Read more

A ‘NICE’ Innovation: The Proportionate Approach
Written by Kiera Lander, Assistant Project Manager The National Institute for Health and Care Excellence (NICE) has long been at the forefront of health technology assessment (HTA), ensuring that patients have access to innovative, ... Read more

Out of the frying pan and into the fire: Moving to a market access consultancy company
Written by Brian O'Toole, HTA Writing Lead Last year I changed jobs. It was a major life decision that took time to make as it meant leaving the comfort and familiarity of academia for the hustle and bustle of a market access ... Read more

AI-enabled triage in the NHS: an economic analysis
Written by Mark Pennington, Consultant Health Economist With the UK’s National Health Service (NHS) facing an unprecedented patient demand for healthcare, there is increasing interest in artificial intelligence (AI) and how it can ... Read more

Modelling requirements for HTA in Europe’s big 5 economies
Written by Oliver Burn, Consultant Health Economist, and Margaret Petrou, Consultant Health Economist Adapting a global economic model for Health Technology Assessment (HTA) submissions in several countries can be challenging, as requirements ... Read more

Advances in artificial intelligence: ChatGPT and the implications for medical writing
Written by Dom Partridge, Medical Writer As artificial intelligence (AI) technology continues to advance, natural language processing (NLP) models like ChatGPT have gained a lot of attention for their ability to generate human-like text. ... Read more
Obtaining a NICE recommendation for your medical device: a view from the external assessors
Written by Mark Pennington, Consultant Health Economist A National Institute for Health and Care Excellence (NICE) recommendation can open the door to widespread adoption for a new medical device. But does getting a NICE recommendation require ... Read more

10 steps for a successful global economic model
Written by Beth Hancock, Senior Consultant Health Economist Pharmaceutical companies often develop global cost-effectiveness and budget impact models, with the aim of adapting them for specific countries. In principle, this is an efficient and ... Read more

Conceptual modelling and NICE requirements: a significant change or hill of beans?
Written by Alec Miners, Head of Health Economics The National Institute for Health and Care Excellence’s (NICE’s) new processes and methods guide includes several important changes to the way submitted economic evaluations should be ... Read more

British Heart Week 2022
As we approach the end of British Heart Week, we reflect on some of the key stats around heart conditions in the UK and our contribution as a company to help bring cutting-edge treatments to patients with cardiovascular diseases Read more

Patient Participation Groups: A voice for patients for 50 years
Written by Dom Partridge, Medical Writer 6th to 13th June 2022 is Patient Participation Group (PPG) Awareness Week; aiming to raise the profile of PPGs within primary healthcare. The first Patient Participation Group The first PPG was set ... Read more

The Innovative Medicines Fund is now available to fast-track non-cancer medicines recommended with managed access
The Innovative Medicines Fund (IMF) launched this month (June 2022) to support faster access to non-cancer drugs. This process involves NHS England and the National Institute for Health and Care Excellence (NICE) working in partnership with pharmaceutical companies to respond to the long-standing challenge of evaluating medicines and addressing the uncertainty around their effectiveness. Read more
NICE tests a new approach for evaluating and reimbursing antimicrobials
Written by Chris Hellmund, Senior Medical Writer Antimicrobial resistance is a major threat to global health that threatens to reverse the progress of modern medicine. More infections are becoming harder to treat, and without coordinated ... Read more

A quick look at network meta-analyses for National Numeracy Day!
Written by Abby Paine, Statistician It’s National Numeracy Day, which is run by National Numeracy, a charity that aims to enable everyone across the UK to be confident and competent in using numbers and data. This is such an important issue; ... Read more

Limiting conference abstract searching for systematic reviews
Written by Isobel Munro, Associate Systematic Review Analyst The purpose of conducting a systematic literature review (SLR) is to identify a complete and unbiased set of studies relevant to the proposed research question. However, it is not ... Read more

Moving from academia to consultancy, is it for you?
Written by Alec Miners, Head of Health Economics Contemplating ‘change’ of any kind can be daunting. Choosing a new career direction is no exception. I joined Source Health Economics as Head of Health Economics in March this year after 16 ... Read more

ILAP breakdown for UK HTAs and market access
Written by Luke Parkes, Assistant Project Manager ILAP remit It has been just over a year since the Medicines and Healthcare Products Regulatory Agency (MHRA) established the Innovative Licensing and Access Pathway (ILAP) in response to ... Read more

Evidence hierarchy in GVDs: the use of non-randomised evidence following NICE’s recent methods update
Written by Pip White, Senior Medical Writer The global value dossier (GVD) is a vital resource to ensure internal strategy alignment and facilitate external communication. As described in our previous blog, GVDs incorporate evidence-based ... Read more

Rare Disease Day 2022 – how NICE is responding to the growing rare disease therapy space
Written by Harry Atkins, Assistant Project Manager Today is Rare Disease Day, a day to raise awareness for people across the world living with a rare disease, their families, and those who care for them. Around 300 million people ... Read more

NICE RWE framework
Written by Amy Crompton, Systematic Review Analyst Previously, we discussed the use of real-world evidence (RWE) in the reimbursement assessment of medical devices. In this latest blog, we present an overview of the RWE framework created by ... Read more
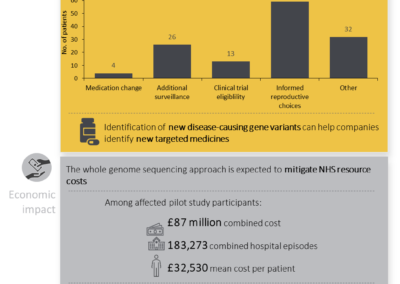
The 100,000 genomes project: paving the way for faster diagnosis and treatment of rare diseases
Written by Emma Lones, Medical Writer What are rare diseases? In the European Union, a rare disease is defined as a disorder affecting ≤5 in 10,000 persons (1). Using this definition, the global population prevalence of rare diseases is ... Read more
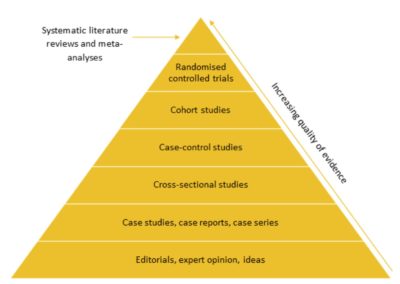
Use of RWE in the reimbursement assessment of medical devices
Amy Crompton (Systematic Review Analyst) and Tom Macmillan (Consultant - Systematic Review) Hierarchy of evidence Different study designs are often ranked in a hierarchy of evidence based on their validity and robustness. Randomised ... Read more

National writing day – connecting patients with new therapies
Written by Dom Partridge, Medical Writer Happy National Writing Day 2021! In line with this year's theme of 'connections', our medical writers have taken a look back at our UK health technology assessments (HTA) submissions over the past ... Read more

Improving your search strategy: date limit filters (2/2)
Amy Cromptom (Systematic Review Analyst) and Tom Macmillan (Consultant - Systematic Review) This blog post follows on from ‘Improving your search strategy: Randomised controlled trial filters’. Electronic database searches can be run on ... Read more

Improving your search strategy: randomised controlled trial filters (1/2)
A search filter is a pre-written search strategy (string of search terms) that is designed to retrieve studies with a particular methodology or focus from a specific database and platform (1). The most common filters are designed to retrieve randomised controlled trials (RCTs), which are widely considered the most robust form of research for evidence-based medicine (2, 3). Read more

Applying health technology assessment requirements to your global value dossier
The global value dossier (GVD) is an essential tool used to internally align the global strategy and externally communicate the value of a product. The GVD presents evidence-based messaging to convey the product value story and is a primary resource for affiliates. Unlike a Health Technology Assessment (HTA) submission, the GVD is strategic, bespoke, and not confined by restrictive templates and requirements. Read more

Collaborative webinar: economic modelling in rare diseases
It can be challenging to develop an economic model in rare diseases that optimises HTA and reimbursement outcomes. It can be difficult to fully capture the effects of the disease and the benefits of treatment whilst using sufficiently robust data to minimise uncertainty. This webinar will introduce a new approach in which multiple companies collaborate to develop a core economic model and common data set. An approach that aims to optimise HTA and reimbursement outcomes for all new treatments in a rare disease. Read more
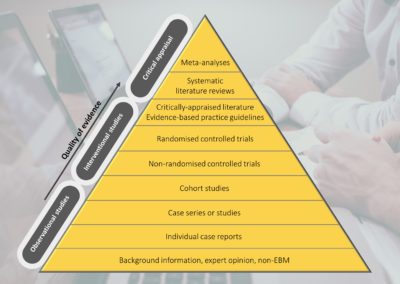
COVID-19, evidence-based medicine and standards for HTA
When Archibald Cochrane formulated his vision for evidence-based medicine (EBM), it was driven by an absence of reliable, robust evidence supporting frequently accepted medical interventions. His observations provoked meticulous evaluations of novel interventions, which subsequently emphasised the need for high-quality evidence in medicine (1). Today, EBM has defined modern medicine; its goal is to minimise the uncertainties in clinical decision-making (2) and to increase the use of high-quality evidence. In practice, this involves the integration of clinical expertise and patient values with the best available evidence (3). Read more
Evidence search for medical devices: challenges and recommendations
In principle, the process of searching for published evidence for medical devices is no different from other health technologies. The NICE process guidelines do not specify alternative sources or a different level of rigour for any kind of health technology. However, when conducting searches for medical devices, the evidence base should be approached differently in order to optimise the precision and sensitivity of the search. Read more

Exploring the NICE MTEP process for developing medical device guidance
The NICE Medical Technologies Evaluation Programme (MTEP) evaluates novel medical devices and diagnostics for use within the National Health Service (NHS). There are currently 3 outputs from the programme: medical technologies guidance (MTGs), diagnostics guidance (DGs) and medical innovation briefings (MIBs). A fourth type of guidance, digital health technologies (DHTs), is being piloted. Read more
The NICE HST process (3/3) – challenges associated with appraising treatments for ultra-rare indications
In previous blog posts, we explored the origins of NICE’s Highly Specialised Technologies (HST) process and compared it with the Single Technology Assessment (STA) process. In this blog, we review the first 10 HST appraisals and identify key issues raised by the Evidence Review Groups (ERGs) Read more

Reporting current research with living systematic literature reviews
Although considered the gold standard of quality research, systematic literature reviews (SLRs) are very resource intensive (e.g. long timeline, high budget) [1]. This, in combination with the broader issue that “research outpaces understanding,” SLRs get quickly out-of-date [2]. Read more

The NICE HST process (2/3) – differences in assessment of cost-effectiveness between the HST and STA
In a previous post, we discussed the origins of the NICE Highly Specialised Technologies (HST) process for the evaluation of therapies for rare diseases. In this blog, we examine the key differences between the Single Technology Appraisal (STA) and the HST processes. There are numerous differences between the two processes; from timescale to individual requirements. Formal guidelines for the HST process are available here. Read more

The NICE HST process (1/3) – origins and current state of affairs
Treatments for very rare conditions represent a unique challenge to payers. Extremely low patient numbers mean that often only Phase 1/2 trial data are available, and that natural history, quality of life and resource use data are limited. Combined with high unit costs, these evidence challenges result in estimates of cost-effectiveness that are subject to a greater degree of uncertainty. The Health Technology Assessment (HTA) process is well-suited to the evaluation of therapies which are expected to benefit large numbers, but concerns exist as to their application to orphan therapies, and whether standard HTA processes accurately reflect societal preferences for treating rare diseases (1). Read more
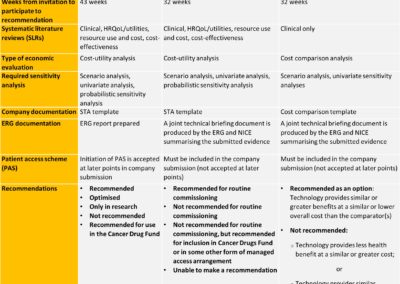
Is the NICE FTA process resulting in faster access to the most cost-effective therapies?
On the 1st April 2017, the National Institute for Health and Care Excellence (NICE) introduced a fast-track appraisal (FTA) process, with the aim of providing quicker access for patients to the most cost-effective new treatments. Two years on, has this objective been achieved? Since the introduction of the FTA process, final FTA guidance has been published for only four technologies (TA486, TA497, TA521 and TA572) [1]. All four therapies were considered based on a cost-comparison. Read more
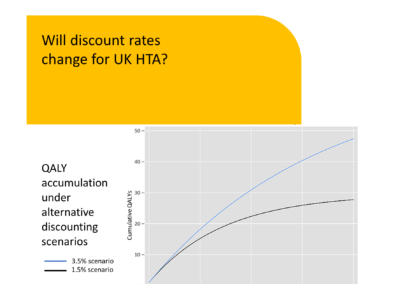
Will discount rates change for UK HTA
Written by David Trueman, Director The rate at which future costs and outcomes are discounted has long stood at 3.5% for UK HTA agencies (1,2). The source of this discount rate is the Social Time Preference Rate (STPR), presented in previous ... Read more

Why multi-company collaboration for HTA evidence generation in rare diseases is a good thing
Project HERCULES is a unique international multi-stakeholder collaborative project set up by Duchenne UK to develop tools and evidence to support Health Technology Assessment (HTA) and reimbursement decisions for new treatments for Duchenne Muscular Dystrophy (DMD). It brings together eight leading pharmaceutical companies, academics, patient organisations and advisers to develop and build a better evidence base for DMD to aid the pricing and reimbursement stage of drug development. Read more

Rapid reviews: what you need to know
Written by Julie Fricke (Systematic Review Analyst) and Neil Webb (Head of Systematic Review) As described in the Cochrane Collaboration Handbook, a systematic literature review (SLR) attempts to collate all empirical evidence that fits ... Read more
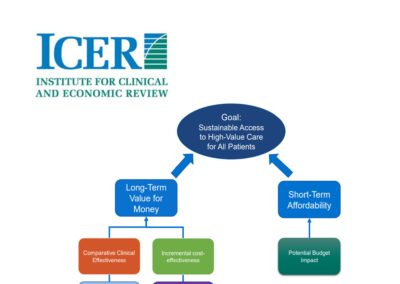
Explaining ICER, America’s answer to NICE
The Boston-based Institute for Clinical and Economic Review, otherwise known as ICER, has caught the attention of important stakeholders in the past few years. Between reports for high-impact drugs that have stirred public opinion (such as a review of Orkambi for cystic fribrosis) and its increasing popularity with healthcare payers, ICER is becoming a key player in transforming the drug-pricing mechanism in the US. Read more

The importance of peer-review for your search strategy
The search strategy is the foundation of a well-conducted systematic literature review (SLR) and is the fundamental element that can affect the overall quality of the results of the final review (1, 2). The aim of a search string is to achieve a balance between sensitivity (capturing all relevant articles) and precision (eliminating irrelevant hits); a lack of balance has implications on the quality of the evidence identified and the resources required to conduct the review. Read more

Mixture cure models: modelling survival when some patients are cured
The standard survival analysis techniques used in oncology modelling, such a parametric survival curves, provide a useful way of estimating transition probabilities to populate economic models. These models are predicated on the idea that at some point all patients will experience the event, be it disease progression or death (more specifically, death due the cancer being studied) and are asymptotic to 0. For many applications this assumption is not problematic. Read more

Duchenne UK’s Project HERCULES awarded prestigious rare disease award
Written by Juliet Mumby-Croft, Director Project HERCULES is a unique international multi-stakeholder collaborative project set up by Duchenne UK to develop tools and evidence to support Health Technology Assessment (HTA) and reimbursement ... Read more

htasurv: a Stata module for performing survival analysis in economic evaluations
Written by David Trueman, Director htasurv is an open source Stata module for assessing alternative parametric distributions when extrapolating survival data for use in health economic models. The function distfind loops through alternative ... Read more

Ten guiding principles for model write-ups and economic sections of HTA submissions
The following pointers aim to provide practical guidance to strengthen the communication of your economic case, avoid the introduction of mistakes and make the deadline. 1. Validate your model first. Then get someone else to validate it. Read more

Model methods: part 2 – finding an elegant solution
When developing a model methodology, there should be a Eureka moment. The moment when you find a model structure that reflects natural history, captures the relative benefits and harms of the new intervention versus standard of care (SoC) and uses the minimum number of assumptions. The methodology chosen should diminish uncertainty and not add to it as a result of burdensome complexity or layers of assumption that render a conclusion almost impossible. Read more

Model methods: part 1 – to include or not to include?
Written by Juliet Mumby-Croft, Director It’s an accepted principle that the structure of a cost-effectiveness model should reflect the natural history of disease. Further, that it should capture the differences in outcome, cost and resource ... Read more