Written by Chris Hellmund, Senior Medical Writer
In a previous post, we discussed the origins of the NICE Highly Specialised Technologies (HST) process for the evaluation of therapies for rare diseases. In this blog, we examine the key differences between the Single Technology Appraisal (STA) and the HST processes. There are numerous differences between the two processes; from timescale to individual requirements. Formal guidelines for the HST process are available here.
Eligibility
As discussed previously, the eligibility criteria are an important consideration when choosing the approach to submitting a technology appraisal. The HST process is only available in very rare conditions and must fulfil the seven defined criteria (listed below).
- The target patient group for the technology in its licensed indication is so small that treatment will usually be concentrated in very few centres in the NHS
- The target patient group is distinct for clinical reasons
- The condition is chronic and severely disabling
- The technology is expected to be used exclusively in the context of a highly specialised service
- The technology is likely to have a very high acquisition cost
- The technology has the potential for life-long use
- The need for national commissioning of the technology is significant
QALY threshold
The QALY threshold is an important difference between the HST and STA processes. In the STA process, the threshold is £20,000-£30,000 per QALY (or £50,000 for therapies meeting the end of life criteria).
In the HST process, the threshold is a cost per QALY of £100,000. For ICERs greater than £100,000 per QALY gained, the committee will consider the size of the therapeutic improvement, using a weighted threshold based on the number of additional QALYs gained (see Table 2).
Table 2: QALY weighting

For example, a QALY gain of 18.3 would be assigned a weighting of 1.83, leading to a threshold of £183,000. The change in the willingness-to-pay (WTP) threshold as a function of incremental QALYs is presented in Figure 1.
Figure 1: Weighted cost-effectiveness threshold
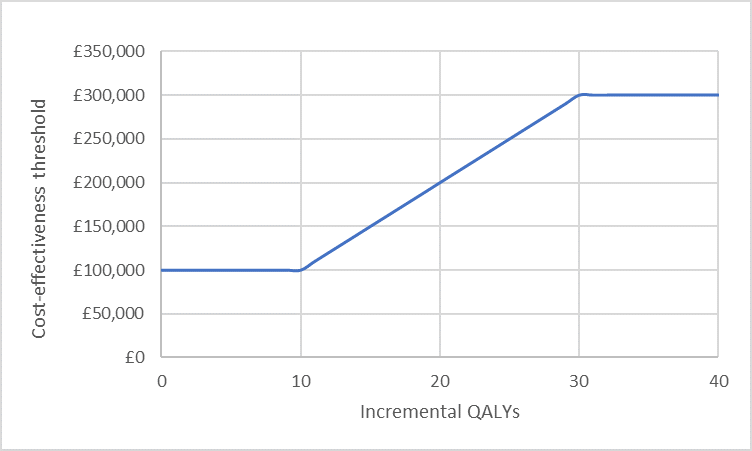
This creates a unique situation in probabilistic sensitivity analysis – in addition to uncertainty in modelled costs and outcomes, the WTP threshold is also subject to uncertainty. Probabilistic sensitivity analysis can be modified so that for each simulation run, the relevant threshold is recorded (based on the undiscounted QALY gain) and a “Yes” or “No” recorded based on whether the simulated ICER falls below that threshold. This allows estimation of the proportion of simulations in which the intervention can be considered cost-effective based on a variable WTP threshold.

NICE’s interim HST guidance does not explicitly state whether the additional QALYs used to define the weighting are discounted or undiscounted, however precedence from previous HST committee meetings suggests that undiscounted QALYs should be used [1].
However, weighting the threshold has been criticised. One criticism is that, since estimating long-term QALY gains usually involves extrapolation of short-term data, the estimated gain may not be robust [2]. Additionally, a threshold which increases with QALY gain is seen by some to conflict with what is known about public preferences. HST guidance is currently under review.
Other considerations
The following considerations may also be more likely in an HST evaluation than in an STA:
- The inclusion of carer disutility, which may increase QALY gains for the intervention
- The application of a 1.5% discount rate for costs and outcomes, which is lower than the 3.5% required in the STA process. This may impact the ICER by increasing the QALY gain, but may harm cost-effectiveness if the intervention has a high upfront cost and long-term costs are avoided.
Decision-making criteria
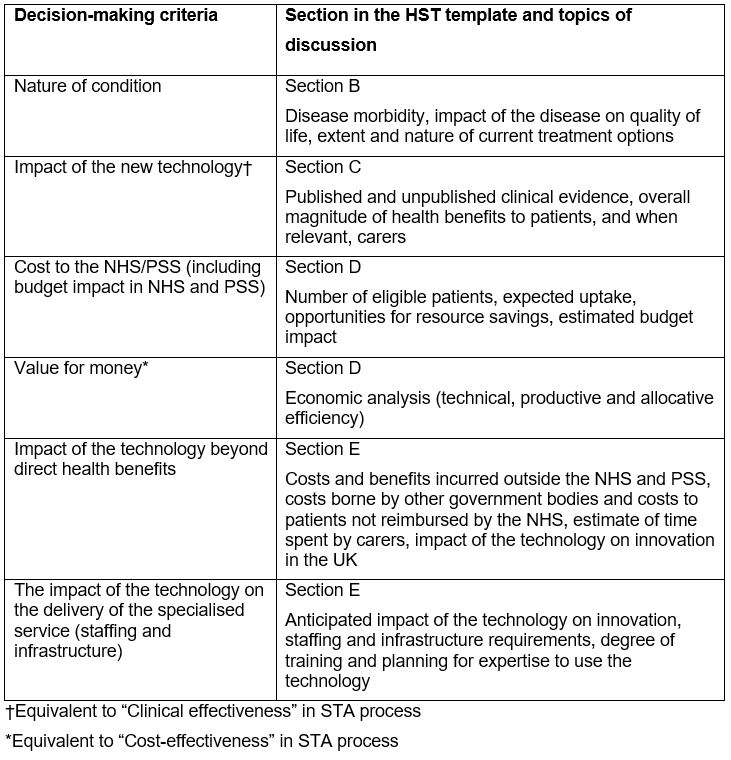
Given the small patient numbers and high upfront cost generally observed with new technologies for rare conditions, the HST decision-making criteria are not solely based on clinical and cost-effectiveness, as is the case for STA submissions. The relevant sections corresponding to the decision-marking criteria are listed in Table 3 below.
Table 3: Decision-making criteria and relevant sections of HST document
Minimum time to decision
An STA process takes between 40 and 50 weeks, depending on whether or not an Appraisal Consultation Document is produced (ACD). The minimum time to decision for an HST appraisal is either 17 weeks (without public consultation) or 27 weeks (with public consultation) and should be issued within four months of EMA marketing authorisation.
Conclusion
The HST process is a novel approach which attempts to formally evaluate often high-cost technologies for small patient groups. The requirements set by NICE have attempted to capture the broader impact of orphan therapies, including societal benefits. The key differences between the HST and STA processes relate to the WTP threshold and the technology’s impact beyond direct health benefits. With a total of 11 successful submissions to date, and several more in development, manufacturers will benefit from understanding the requirements for bringing their highly specialised technologies to the UK market.
References
- National Institute for Health and Care Excellence. Strimvelis for treating adenosine deaminase deficiency–severe combined immunodeficiency – public committee slides (Slide 23). 2017; Available from: https://www.nice.org.uk/guidance/hst7/documents/1
- https://blogs.bmj.com/bmj/2017/04/18/nices-proposed-new-qaly-modifier-for-appraising-highly-specialised-technologies/




