Written by Chris Hellmund, Senior Medical Writer
Origins of the Highly Specialised Technologies (HST) process
Treatments for very rare conditions represent a unique challenge to payers. Extremely low patient numbers mean that often only Phase 1/2 trial data are available, and that natural history, quality of life and resource use data are limited. Combined with high unit costs, these evidence challenges result in estimates of cost-effectiveness that are subject to a greater degree of uncertainty.
The Health Technology Assessment (HTA) process is well-suited to the evaluation of therapies which are expected to benefit large numbers, but concerns exist as to their application to orphan therapies, and whether standard HTA processes accurately reflect societal preferences for treating rare diseases (1). Technologies for rare diseases are rarely cost-effective at standard thresholds for cost-effectiveness, but in recent years, global orphan drug production has risen exponentially, and is not expected to slow down (2).
The Health and Social Care Act 2012 transferred responsibility for evaluating high cost drugs for rare conditions in England to the National Institute for Health and Care Excellence (NICE), through the establishment of its Highly Specialised Technologies (HST) process (3). Other agencies worldwide follow various frameworks to evaluate therapies for rare diseases:
- The Canadian Agency for Drugs and Technologies in Health (CADTH) uses the same process in common and rare diseases.
- The Scottish Medicines Consortium established:
- The Patient & Clinician Engagement (PACE) process in 2014, which includes the added social value of orphan drugs; and
- the ultra-orphan process in 2019, which assesses orphan drugs and includes a real-world evidence generation step.
- In the US, the Institute for Clinical and Economic Review (ICER) applies a modified value assessment framework for treatments for ultra-rare diseases, which accounts for the societal value of a new technology (4).
- Scotland, France and Germany all have special HTA considerations which apply to orphan drugs (5).
Evaluation criteria
NICE recognises in its interim HST process and methods guide that “a simple utilitarian approach, in which the greatest gain for the greatest number is valued highly, is unlikely to produce guidance which would recognise the particular circumstances of these very rare conditions.” Accordingly, in its deliberations, the HST committee considers a broad range of factors including the nature of the condition, clinical effectiveness, value for money, and the impact of the technology beyond direct health benefits (6).
Topics evaluated through the HST programme are formally referred to NICE by ministers, providing they meet all criteria listed below.
- The target patient group for the technology in its licensed indication is so small that treatment will usually be concentrated in very few centres in the NHS;
- The target patient group is distinct for clinical reasons;
- The condition is chronic and severely disabling;
- The technology is expected to be used exclusively in the context of a highly specialised service;
- The technology is likely to have a very high acquisition cost;
- The technology has the potential for life-long use;
- The need for national commissioning of the technology is significant.
Willingness to pay threshold in the HST process
In 2017, NICE introduced a £100,000 willingness to pay (WTP) threshold for therapies that deliver fewer than 10 QALYs to the patient in their lifetime, which can rise to £300,000 for treatments that deliver more than 30 additional QALYs to the patient in their lifetime (6). This is 10–15 times the £20,000–£30,000 threshold in the Single Technology Appraisal (STA) process. There has been criticism of the lack of rationale for the £100,000 threshold and the QALY modifier given the available evidence on public preferences – an online discrete choice survey of 3,669 members of the UK population revealed that respondents preferred to treat patients with larger QALY gains, but at a diminishing rate, suggesting a preference to disperse QALY gains rather than concentrate them in a small population (7)(8).
If the ICER exceeds £100,000 and the QALY gains aren’t sufficient to raise the threshold, the recommendation may include a managed access agreement (MAA), which allows for restricted availability of the drug on the NHS while real world data are collected to address uncertainties in the evidence base (8).
HST submissions to date
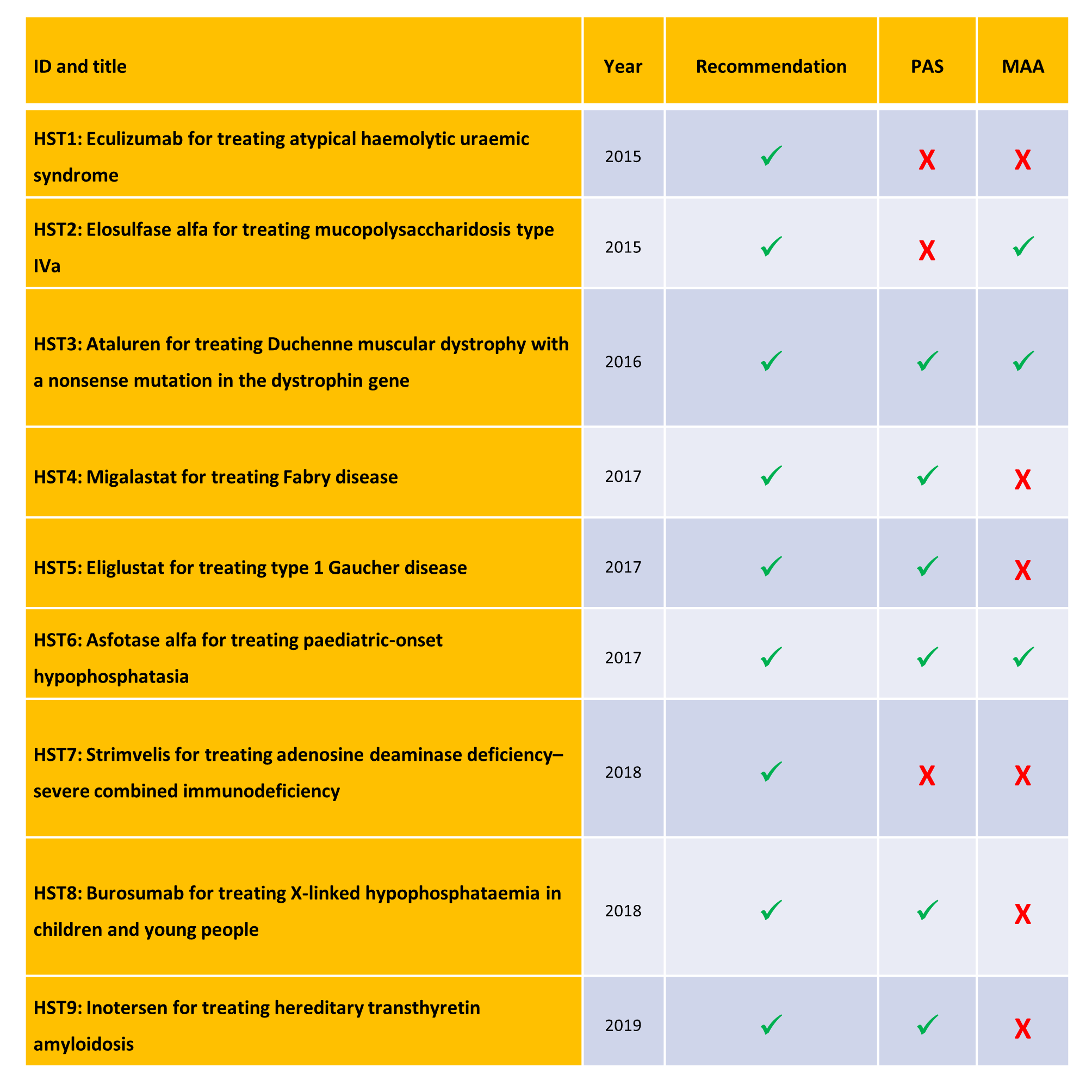
Since the HST process was introduced in 2013, only nine recommendations have been published (see the table below). Although all of these have received a positive recommendation, three have included an MAA (a conditional ‘yes’), and six have included a patient access scheme (PAS).
Managed Access Agreements (MAA)
Managed access agreements are expected to be more common in the HST process, as they provide access to patients who are most in need of treatment whilst minimising the risk to NHS finances in the face of uncertainty about cost-effectiveness. However, the negotiation of complex agreements, and the need for additional committee meetings for clarification on cost, have been criticised for slowing down the process . This is problematic, as the HST process is intended to accelerate access to treatments for diseases that are often fast-progressing, and which subject decision-makers to the political pressure of addressing an unmet need.(9,10)
What’s next?
To date, relatively few interventions have been appraised via the HST process. The process and methods are still evolving: the £100,000 threshold and QALY modifier were introduced only two years ago, and the process and methods guides have yet to be finalised (6).
In 2019/20, NICE will be undertaking a review of the process and methods of the HST programme, through consultation with industry, the public, and other stakeholders (11). It hoped that this will lead to more complete guidance for submitting manufacturers.
References
- Drummond M, Wilson D, Kanavos P, Ubel P, Rovira J. Assessing the economic challenges posed by orphan drugs. Int J Technol Assess Health Care. 2007;23(1):36–42.
- EvaluatePharma. Orphan Drug Report 2019 [Internet]. 2019. Available from: https://www.evaluate.com/thought-leadership/pharma/evaluatepharma-orphan-drug-report-2019
- Health and Social Care Act 2012 [Internet]. [cited 2019 May 31]. Available from: http://www.legislation.gov.uk/ukpga/2012/7/contents/enacted
- Institute for Clinical and Economic Review. Orphan Drug Assessment: Final Framework Adaptations [Internet]. 2017. Available from: https://icer-review.org/material/final-ultra-rare-adaptations/
- Heyes A, McBride D, Pearson I, Copley-Merriman K. HTA and Reimbursement Considerations for Rare Disease in European Markets: What are the implications for manufacturers? In Barcelona; 2018. Available from: https://www.rtihs.org/sites/default/files/29385%20Heyes%202018%20HTA%20and%20reimbursement%20considerations%20for%20rare%20diseases%20in%20European%20markets%20what%20are%20the%20implications%20for%20manufacturers.pdf
- National Institute for Health and Care Excellence,. Interim Process and Methods of the Highly Specialised Technologies Programme Updated to reflect 2017 changes [Internet]. 2017 [cited 2019 May 31]. Available from: https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-highly-specialised-technologies-guidance/HST-interim-methods-process-guide-may-17.pdf
- Rowen D, Brazier J, Mukuria C, Keetharuth A, Risa Hole A, Tsuchiya A, et al. Eliciting Societal Preferences for Weighting QALYs for Burden of Illness and End of Life. Med Decis Mak Int J Soc Med Decis Mak. 2016;36(2):210–22.
- Harvey, E. Can managed access agreements help unlock access through the NICE Highly Specialised Technology Appraisal Process? [Internet]. 2018 [cited 2019 May 31]. Available from: https://pharmaphorum.com/market-access-2/can-managed-access-agreements-help-unlock-access-through-the-nice-highly-specialised-technology-appraisal-process/
- Barham, L. NICE and Highly Specialised Technologies: three years on [Internet]. Pharmaphorum. 2016 [cited 2019 Jun 20]. Available from: https://pharmaphorum.com/views-and-analysis/nice-and-highly-specialised-technologies-three-years-on/
- Mcintosh, E. Less speed, more HST [Internet]. 2018 [cited 2019 Jun 20]. Available from: https://www.incisivehealth.com/less-speed-more-hst/
- Department of Health and Social Care,, Association of the British Pharmaceutical Industry,. The 2019 voluntary scheme for branded medicines pricing and access: chapters and glossary [Internet]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/761834/voluntary-scheme-for-branded-medicines-pricing-and-access-chapters-and-glossary.pdf




