Written by Emma Lones, Medical Writer
What are rare diseases?
In the European Union, a rare disease is defined as a disorder affecting ≤5 in 10,000 persons (1). Using this definition, the global population prevalence of rare diseases is estimated to be between 3.5–5.9% (excluding rare cancers, infectious diseases, and poisoning) (2). This is equivalent to 263–446 million persons affected globally at any point in time. Most patients with a rare disease remain without a molecular diagnosis after standard diagnostic testing (3-5). On average, it takes 5.6 years for patients with a rare disease to receive a proper diagnosis in the UK, with each patient visiting four primary care physicians, four specialists, and receiving two to three misdiagnoses (6).
Rare diseases are both disabling and costly. Factors contributing to the higher cost of care compared with more common diseases include: a greater number of diagnostic tests, more expensive diagnostic tests, more visits to specialists, greater need for mental health support, and more expensive treatments (6).
What is the 100,000 genomes project?
The 100,000 genomes project was launched in 2013 by the UK government. The project aimed to sequence 100,000 whole genomes from National Health Service (NHS) patients to study rare diseases, cancers and infections. The study enrolled patients who had been identified by healthcare professionals and researchers as having a rare disease that had not been diagnosed by usual care in the NHS. A broad range of rare disease categories were covered, where either no diagnostic tests were available or approved diagnostic tests did not include genome sequencing as part of usual care. As most rare diseases are inherited, the genome of the affected individual (the proband) plus close blood relatives were included, where possible. The project collects clinical as well as genetic data, following patients over the course of their lifetime using electronic health records. The results of a pilot study investigating the effect of the whole genome sequencing approach on the genetic diagnosis of rare diseases in the NHS are now available (7).
Study results
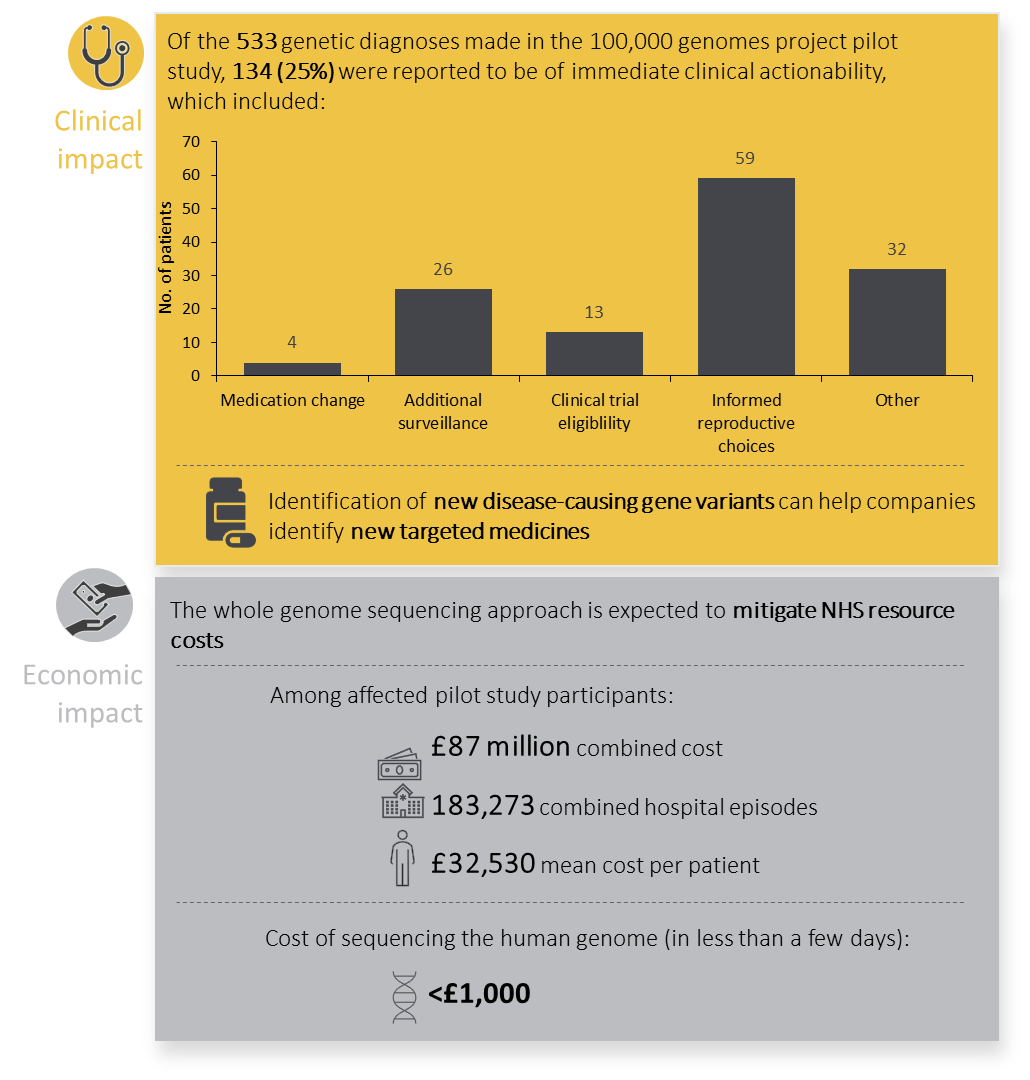
A total of 4,660 participants (2,183 probands and 2,477 family members) were included in the pilot study. Among the study participants, 161 disorders across a broad spectrum of rare diseases were present. Genetic diagnoses were made in 25% of probands, of which 14% were found in regions of the genome that would have been missed by conventional methods. Of the genetic diagnoses made, 25% had immediate ramifications for clinical decision making for probands or their relatives. Diagnostic yields were much higher in larger family structures, and for disorders likely to be caused by a single gene rather than disorders which are likely to have a complex cause (35% vs. 11%, respectively). In addition, numerous novel disease gene-associations were identified (three confirmed and 19 with compelling evidence, likely to be confirmed in independent datasets).
Implications for medical care
The 100,000 genomes project is the first study to use whole genome sequencing in a healthcare system for large numbers of patients. This pilot study paves the way for this technique to be used in the NHS and worldwide with the potential to revolutionise prompt access to treatments for rare diseases.
If you are interested in finding out more about our market access services, particularly in rare diseases, please contact the Market Access and Value Communication team at Source Health Economics. Source is an independent consultancy specialising in evidence generation, health economics, and communication.
- European Commission. Rare diseases. Available at:https://ec.europa.eu/health/non_communicable_diseases/rare_diseases_en[Accessed December 2021]. 2021.
- Nguengang Wakap S, Lambert DM, Olry A, Rodwell C, Gueydan C, Lanneau V, et al. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet. 2020;28(2):165-73.
- Boycott KM, Rath A, Chong JX, Hartley T, Alkuraya FS, Baynam G, et al. International cooperation to enable the diagnosis of all rare genetic diseases. Am J Hum Genet. 2017;100(5):695-705.
- Taylor JC, Martin HC, Lise S, Broxholme J, Cazier JB, Rimmer A, et al. Factors influencing success of clinical genome sequencing across a broad spectrum of disorders. Nat Genet. 2015;47(7):717-26.
- Splinter K, Adams DR, Bacino CA, Bellen HJ, Bernstein JA, Cheatle-Jarvela AM, et al. Effect of genetic diagnosis on patients with previously undiagnosed disease. N Engl J Med. 2018;379(22):2131-9.
- globalgenes.org. Rare disease impact report: Insights from patients and the medical community. Available at:https://globalgenes.org/wp-content/uploads/2013/04/ShireReport-1.pdf[Accessed November 2021]. 2013.
- Genomes Project Pilot Investigators, Smedley D, Smith KR, Martin A, Thomas EA, McDonagh EM, et al. 100,000 genomes pilot on rare-disease diagnosis in health care – preliminary report. N Engl J Med. 2021;385(20):1868-80.