Written by Neil Webb (Head of Systematic Review) and Chris Hellmund (Senior Medical Writer)
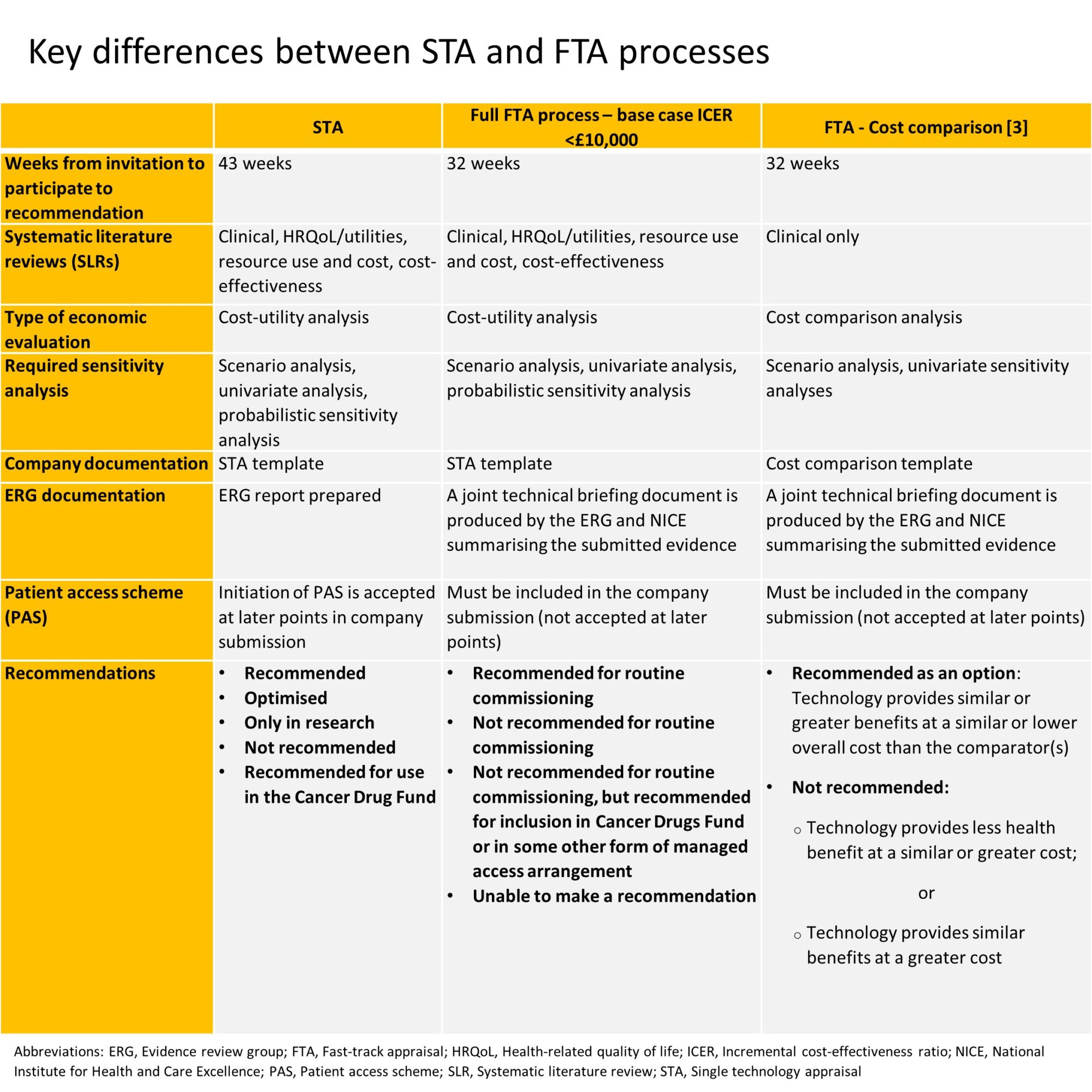
On the 1st April 2017, the National Institute for Health and Care Excellence (NICE) introduced a fast-track appraisal (FTA) process, with the aim of providing quicker access for patients to the most cost-effective new treatments. Two years on, has this objective been achieved?
All technology appraisals are candidates for the FTA process, providing they fulfil the following criteria [1]:
- The company’s base-case incremental cost-effectiveness ratio (ICER) is less than £10,000 per quality-adjusted life year (QALY) gained.
It is likely that the most plausible ICER is less than £20,000 per QALY gained, and it is highly unlikely that it is greater than £30,000 per QALY gained.
or
- A cost comparison case can be made that shows the intervention is likely to provide similar or greater health benefits at similar or lower cost than technologies already recommended in technology appraisal guidance for the same indication.
Since the introduction of the FTA process, final FTA guidance has been published for only four technologies (TA486, TA497, TA521 and TA572) [1]. All four therapies were considered based on a cost-comparison.
No technologies that offer exceptional value for money (ICER < £10,000/QALY) have been evaluated through the FTA process, although a recent article suggested that 26 therapies evaluated through the Single Technology Appraisal (STA) process between 2006 and 2016 would have satisfied the criteria. By extrapolation, it is estimated that 15% of therapies evaluated by NICE would be expected to meet these criteria [2].
For the four therapies that were reviewed based on a cost comparison, a recommendation was published on average within 36 weeks. This compares with an average 43 weeks for the NICE STA process. However, with each FTA submission, the interval between invitation to participate and recommendation has increased, becoming more distant from the 32-week target (34 weeks for first FTA [TA486] and 41 weeks for latest FTA [TA572]) [1].
The evidence to date suggests that the FTA process is being under-utilised, potentially delaying access to the most cost-effective new treatments. Also, though based on a very small sample size, it appears that the target of 32 weeks from invitation to participate to a published recommendation is not always met.