Written by Oliver Burn, Consultant Health Economist, and Margaret Petrou, Consultant Health Economist
Adapting a global economic model for Health Technology Assessment (HTA) submissions in several countries can be challenging, as requirements can vary considerably between countries. It is important to keep differing country requirements in mind when designing a global model, to ensure it is both easily adaptable to different countries, and to avoid pitfalls, such as missing a key component required for a successful HTA submission.
We have compared the HTA requirements and methodologies across Europe’s big 5 economies (UK [England and Wales, and Scotland]), France, Germany, Italy and Spain) (1-6). Criteria are predominantly focused on methods that would require structural adaptations within a model:
-
-
- Perspective
- Sensitivity analysis requirements
- Utilities (inclusion of carer utility)
- Threshold
- Severity modifiers
-
Our findings are summarised in Table 1 and detailed below.
Each country requires a different model perspective, therefore models should be easily adaptable to include different types of costs, such as societal costs. One-way, probabilistic, and scenario analyses are generally required for all countries, however models need to also be adaptable to use additional outcomes, such as net health benefit and to include functionality for probabilistic scenario analysis. EQ-5D is the most commonly preferred utility measure, and although carer quality of life (QoL) is not discussed by all countries, it is generally accepted if there is strong justification. Many countries do not have formal willingness to pay thresholds or severity modifiers; however, to accommodate those that do, the calculations for severity modifiers and flexibility for the inclusion of thresholds should be incorporated.
In summary, it is important to bear in mind what will be required early on in global model development to allow flexibility for all countries in which reimbursement will be pursued. This will help to avoid unnecessarily long adaptation times during submission. More information on the steps for a successful global model can be found here.
Table 1: HTA guidance summary
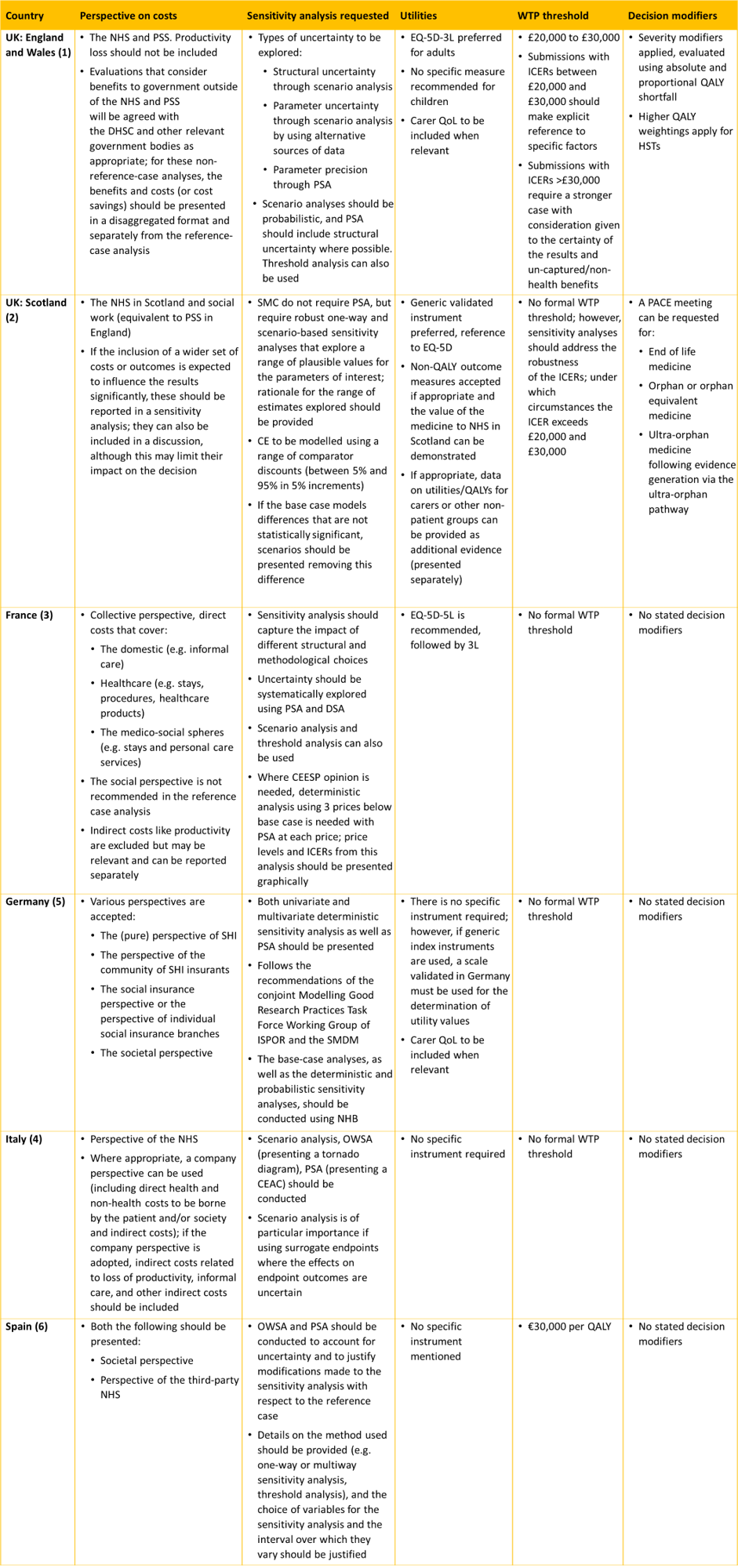
Abbreviations: CE, cost-effectiveness; CEAC, cost-effectiveness acceptability curve; CEESP, Commission on Environmental, Economic and Social Policy; DHSC, Department of Health and Social Care; DSA, deterministic sensitivity analysis; HST, highly specialised technology; ICER, incremental cost-effectiveness ratio; ISPOR, International Society for Pharmacoeconomics and Outcomes; NHB, net health benefit; NHS, National Health Service; OWSA, one-way sensitivity analysis; PACE, Patient and Clinician Engagement; PSA, probabilistic sensitivity analysis; PSS, Personal Social Services, QALY, quality adjusted life year; QoL, quality of life; SHI, statutory health insurance; SMC, Scottish Medicines Consortium; SMDM; Society for Medical Decision Making; WTP, willingness to pay
References
- National Institute for Health and Care Excellence. NICE health technology evaluations: the manual. 2022.
- Scottish Medicines Consortium. Guidance to submitting companies for completion of New Product Assessment Form (NPAF). Available at: https://wwwscottishmedicinesorguk/making-a-submission/. 2022.
- Haute Autorite de Sante. Choices in methods for economic evaluation – HAS. Available at https://wwwhas-santefr/upload/docs/application/pdf/2020-11/methodological_guidance_2020_-choices_in_methods_for_economic_evaluationpdf 2020.
- Agenzia Itaiana del farmaco. PER LA COMPILAZIONE DEL DOSSIER A SUPPORTO DELLA DOMANDA DI RIMBORSABILITÀ E PREZZO DI UN MEDICINALE. Available at https://wwwaifagovit/documents/20142/1307543/20210122_estratto_linee_guida_sezione_Epdf. 2019.
- IQWIG. General Methods. Available at https://wwwiqwigde/methoden/general-methods_version-6-1pdf 2022.
- López-Bastida J, Oliva J, Antonanzas F, García-Altés A, Gisbert R, Mar J, et al. Spanish recommendations on economic evaluation of health technologies. The European Journal of Health Economics. 2010;11(5):513-20.
If you would like to learn more about global economic models or discuss a current challenge, please contact us at Source Health Economics, an HEOR consultancy specialising in evidence generation, health economics, health technology assessment, and value communication.