Written by Elise Elvers, Health Economist
The Boston-based Institute for Clinical and Economic Review, otherwise known as ICER, has caught the attention of important stakeholders in the past few years. Between reports for high-impact drugs that have stirred public opinion (such as a review of Orkambi for cystic fribrosis) and its increasing popularity with healthcare payers, ICER is becoming a key player in transforming the drug-pricing mechanism in the US.
ICER was founded in 2006 by Steven Pearson MD, president of the institute to this day, and initially generated evidence solely on healthcare costs. In 2015, ICER reviewed a new class of cholesterol-lowering injectables and found them to be vastly overpriced (67% higher than the list price). Since then, it has established itself as an independent watchdog for drug prices in the US and reports on a number of technologies each year.
Unlike the UK, the US has no formal cost evaluation framework or single health technology assessment (HTA) body to support healthcare decision-making. The Food and Drug Administration (FDA) evaluates effectiveness and safety of drug candidates only, whilst Medicare is banned from direct negotiation with manufacturers or from using the QALY in cost-effectiveness reviews. This decentralized system, coupled with the lack of drug-pricing regulation, has resulted in soaring costs. The US has the highest (and fastest growing) pharmaceutical costs in the world (1), and an independent assessor of cost-effectiveness could have a significant impact on the affordability of interventions, particularly breakthrough and orphan drugs.
ICER’s goal is not unlike that of NICE in the UK, CADTH in Canada and PBAC in Australia: to provide a transparent and inclusive assessment of cost-effectiveness to determine value-based prices for reimbursement. ICER, however, is an independent consultancy and is funded by non-governmental, non-profit organisations (such as a $14 million grant from the Laura and John Arnold Foundation in 2017) for the publication of its reports. As a result, the institute has been called out for ‘cherry picking’ high impact drugs and for a lack of transparent model methods (2).
How it works
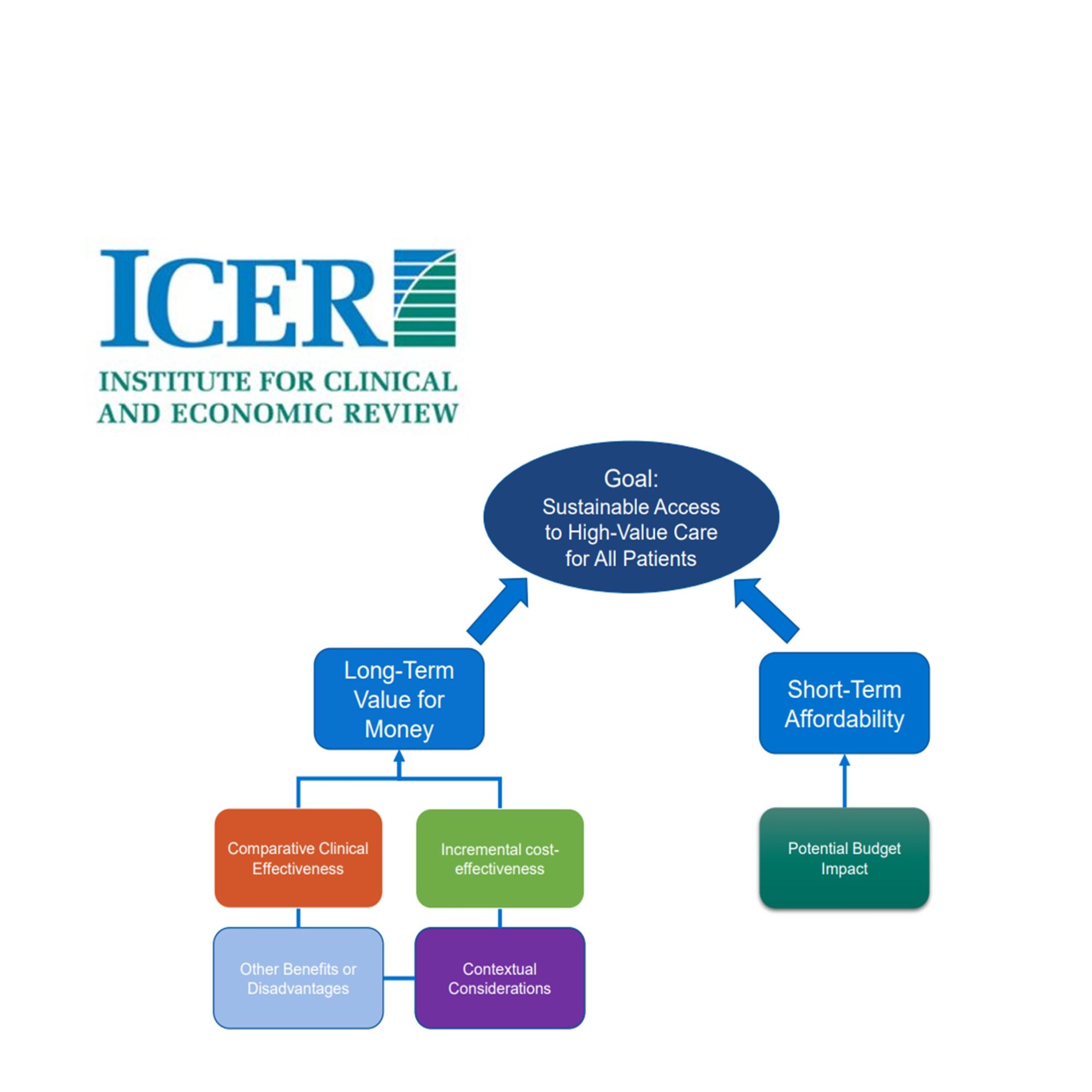
As opposed to other HTA bodies, ICER is powered by a small team of health economists and frequently collaborates with outside academic groups and clinical epidemiologists to ensure best practices (like Skaggs School of Pharmacy and CHOICE at the University of Washington). ICER’s process relies on its value assessment framework (below). The threshold for cost-effectiveness ranges from $50,000 to $100,000 per QALY (3), with special adaptations for ultra-rare diseases and potential cures. Transparency lies at the centre of its activities, with patient groups and manufacturers consulted throughout the process.
After the publication of a draft report to its website (which tend to coincide with FDA approval dates), and a public comment period, ICER and its collaborators congregate for a public meeting in one of 3 locations (the Midwest, California or New England) for an open discussion, presentation and vote on the quality of the evidence put forward for the technology. The members of the voting panel are selected from public health, academia, epidemiology and consumer/patient engagement professions. The meeting is streamed online and attracts payers, patients and their advocacy groups, as well as drug manufacturers. The outcome of the public meeting is then integrated into the final published report.
Does it work?
The question remains whether ICER’s independent status and methodology are sufficient to make a noticeable difference in US decision-making. The Veterans Affairs and CVS already have agreements in place for price negotiations, and a 2016 survey found that 59% of decision makers had used ICER reports as part of the drug evaluation and/or coverage policy process (4). However, concerns remain as to the methodology and public reception of their reports. ICER’s model methods have been extensively criticised for lack of replicability and the absence of a formal external review process (5). Despite its growing influence, analysts highlight the importance of openness and accountability as the institute expands.
References:
- Schumock G, Li E, Suda K, et al. National trends in prescription drug expenditures and projections for 2016—projecting future drug expenditures. Am J Health Syst Pharm. 2016;73:e357–73.
- http://phrma-docs.phrma.org/files/dmfile/Milliman-Critique-of-ICER-CEA-Report-09-19-18.pdf
- http://icer-review.org/wp-content/uploads/2017/06/ICER-value-assessment-framework-Updated-050818.pdf
- Dymaxium Inc. (2016) AMCP Science and Innovation Webinar: Using the AMCP eDossier System’s New Features to Close the Information Gap Between Decision Makers and Manufacturers. June 29. 2016. Available at: http://www.amcp.org/Newsletter.aspx?id=20912&utm_content=bufferd9c98&utm_medium=social&utm_source=linkedin.com&utm_campaign=buffer
- Langley, P. Alternative Facts and the ICER Proposed Policy on Access to Imaginary Pharmacoeconomic Worlds, INNOVATIONS in Pharmacy, 2018. Available at: https://pubs.lib.umn.edu/index.php/innovations/article/download/926/1005/
Further reading:
Payers’ Use of Independent Reports in Decision Making – Will There Be an ICER Effect?, Dymaxium report, March/April 2017
Value Frameworks: Will They Work in the U.S.? What are Stakeholders Saying?, Evidera, November 2017
America’s “NICE?”, Peter J. Neumann, Joshua T. Cohen, Health Affairs, March 2018
Is ICER NICEr?,, Deborah Freund, Jennifer Choi, PharmacoEconomics (2018) 36:385–386, February 201