Written by David Trueman, Director
The rate at which future costs and outcomes are discounted has long stood at 3.5% for UK HTA agencies (1,2). The source of this discount rate is the Social Time Preference Rate (STPR), presented in previous versions of the Treasury’s Green Book, which provides central government guidance on appraisal evaluation (3). The Green Book was updated in March 2018, and with it came new guidance on discounting of health benefits which, if adopted by HTA agencies, may have significant consequences for the outcomes of health technology appraisals.
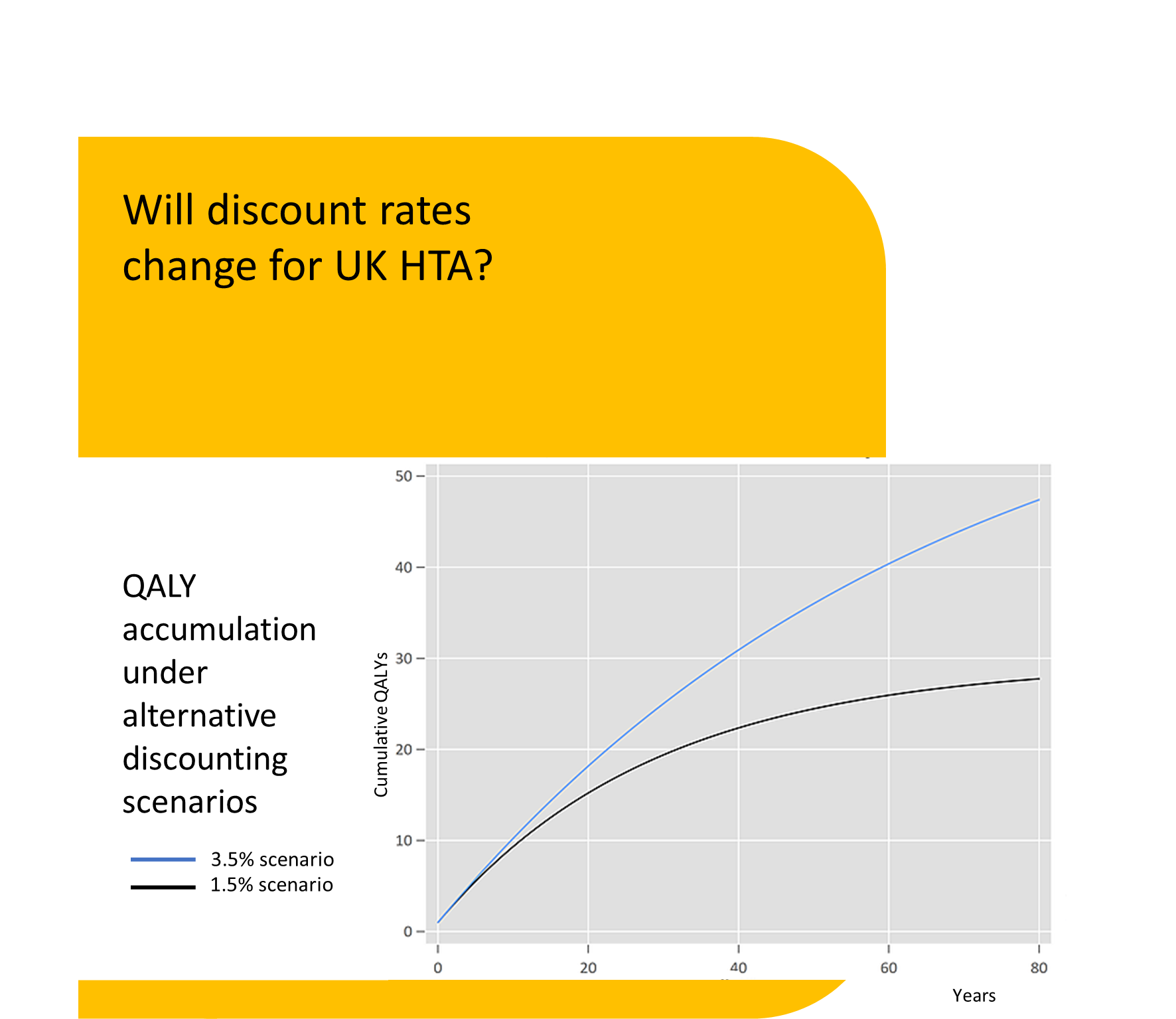
Discounting of resources relating to health and life issues is carried out using the appropriate standard discount rate of 3.5% declining after 30 years. The value of VPFs, SLYs and QALY effects should be discounted at the health rate of 1.5%, declining after 30 years (3).
By mandating that future health effects such as QALYs are discounted at a lower rate than future costs, the new guidance reignites an old debate in the health economics literature. Currently, few countries prescribe the use of differential discounting; the UK reversed differential discounting a decade ago, resulting in a stream of research interest on the subject.
The choice of common vs different discount rates for costs and outcomes depends on multiple factors: whether the social objective is to maximise discounted health outcomes or the present consumption value of health; whether the budget for health care is fixed; the expected growth in the cost-effectiveness threshold; and the expected growth in the consumption value of health (4).
The rationale for the change in the approach adopted by the Green Book is that the ‘wealth effect’ (or real per capita consumption growth element of the discount rate) is now being excluded for health and life effects. Marginal utilities will not be diminishing; as real incomes rise, the utility associated with additional years no longer declines (3).
More generally though, in the technology appraisal process we implicitly equate the costs incurred with health benefits foregone elsewhere in the health system. Therefore, if such a change were to be made in the methods of technology appraisal this may come as something of a surprise to health economists.
The practical implications for the appraisal of many new technologies would be significant. Technologies such as CAR-T and emerging gene therapies are associated with relatively large upfront costs, with benefits accrued over a very long period of time. Discounting these future benefits at a lower rate than costs will make these technologies appear more cost-effective at a given price. Whether or not UK HTA agencies follow suit and update their guidance in line with the Green Book remains to be seen.
- Scottish Medicines Consortium. Working with SMC – A Guide for manufacturers. SMC Secretariat; 2015.
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal [Internet]. NICE; 2014. Available from: nice.org.uk/process/pmg19
- HM Treasury. The Green Book: central government guidance on appraisal and evaluation [Internet]. 2018 [cited 2019 Jan 9]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/685903/The_Green_Book.pdf
- Claxton K, Paulden M, Gravelle H, Brouwer W, Culyer AJ. Discounting and decision making in the economic evaluation of health-care technologies. Health Econ. 2011 Jan;20(1):2–15.