Written by Louisa Rutherford, Senior Systematic Review Analyst
This blog explores the intersection of climate change and the health technology assessment (HTA) process. It examines how incorporating environmental impact data into the HTA process could help healthcare systems achieve their sustainability goals. By integrating environmental considerations into the evaluation of health technologies, decision-makers can promote the adoption of more sustainable practices and technologies, ultimately contributing to global climate targets. To this end, this blog will discuss:
- An overview of the current state of climate change and its implications for healthcare.
- The specific ways in which the HTA process can evolve to include environmental data.
- Advantages and challenges of this integration, and insights into how HTA agencies worldwide are beginning to address these issues.
Overview of the current state of climate change and its implications for sustainability in healthcare
Climate change is a global challenge that impacts every sector, including healthcare. The increasing levels of greenhouse gases (GHGs) are driving significant changes in our climate, which pose serious risks to public health and the ecosystems we depend on (1).
All industries, including healthcare, emit GHGs. In 2014, it was estimated that healthcare produced 4.4% of global net carbon dioxide (CO2) emissions (2). In England, it is estimated that NHS England contributes to 4% of the country’s GHG emissions, with the estimated contribution of the pharmaceuticals industry being 20% (3, 4).
Strategies to reduce and manage the risks of climate change have been agreed on during landmark conferences hosted by the United Nations Framework Convention on Climate Change (UNFCC). Figure 1 shows a timeline summarising the conferences and Intergovernmental Panel in Climate Change (IPCC) reports within the last three decades.
Figure 1: Timeline summarising landmark COPs and publications

COP, Conference of Parties; IPCC, Intergovernmental Panel in Climate Change; NHS, National Health Service.
COP26 in Glasgow 2021 saw 36 countries making commitments to develop low-carbon health systems with a further 14 countries, including the UK, setting a target for a net-zero health service by 2050 (5). If these targets are to be met, healthcare decision-makers will need to consider and understand the environmental impact of products and services used by their health services. In England, this includes the environmental impact from procured health technologies approved via the HTA process.
With the knowledge that health technologies approved by the HTA process become integrated into everyday clinical practice, if technologies with low environmental impact are taken forward, this will help the health sector achieve its net zero targets with positive knock-on effects at a local and, ultimately, a global level.
How the HTA process could evolve to address sustainability in healthcare
Recently published literature discussing health technology assessment (HTA) methodology has included discussion on how environmental data could be included in the HTA decision-making process and which HTA organisations are making progress in this area (6-10). Suggested approaches to incorporate environmental data include:
- Publishing environmental impact data that is already publicly available without further assessment, so it sits alongside an existing cost-effectiveness analysis.
- Carrying out further analyses of environmental impact data that is presented alongside clinical and cost outcomes, but it is not incorporated into the cost-effectiveness model.
- Developing or identifying new models that allow clinical, cost, and environmental data to be assessed in a single quantitative analysis.
An example of where an environmental focused evaluation has been carried out on a technology is with inhalers for managing asthma. Metered dose inhalers and breath-actuated inhalers contain a propellant gas that contributes to climate change. Each puff of some inhalers can produce as much carbon dioxide equivalent (CO2e) in kilograms, as a 115-mile petrol car journey (35 kg CO2e). In contrast, dry powder inhalers or soft mist inhalers produce less than 1kg CO2e per dose. Carbon dioxide equivalent is the unit of measurement for the warming effect of GHGs. In essence it is the exchange rate of other GHGs to carbon and allows comparison of the effect between different GHGs. NICE has produced a decision aid so that patients can now make a choice about which type of inhaler to use (11).
Advantages and challenges of this integration and insights into how HTA agencies worldwide are beginning to address these issues
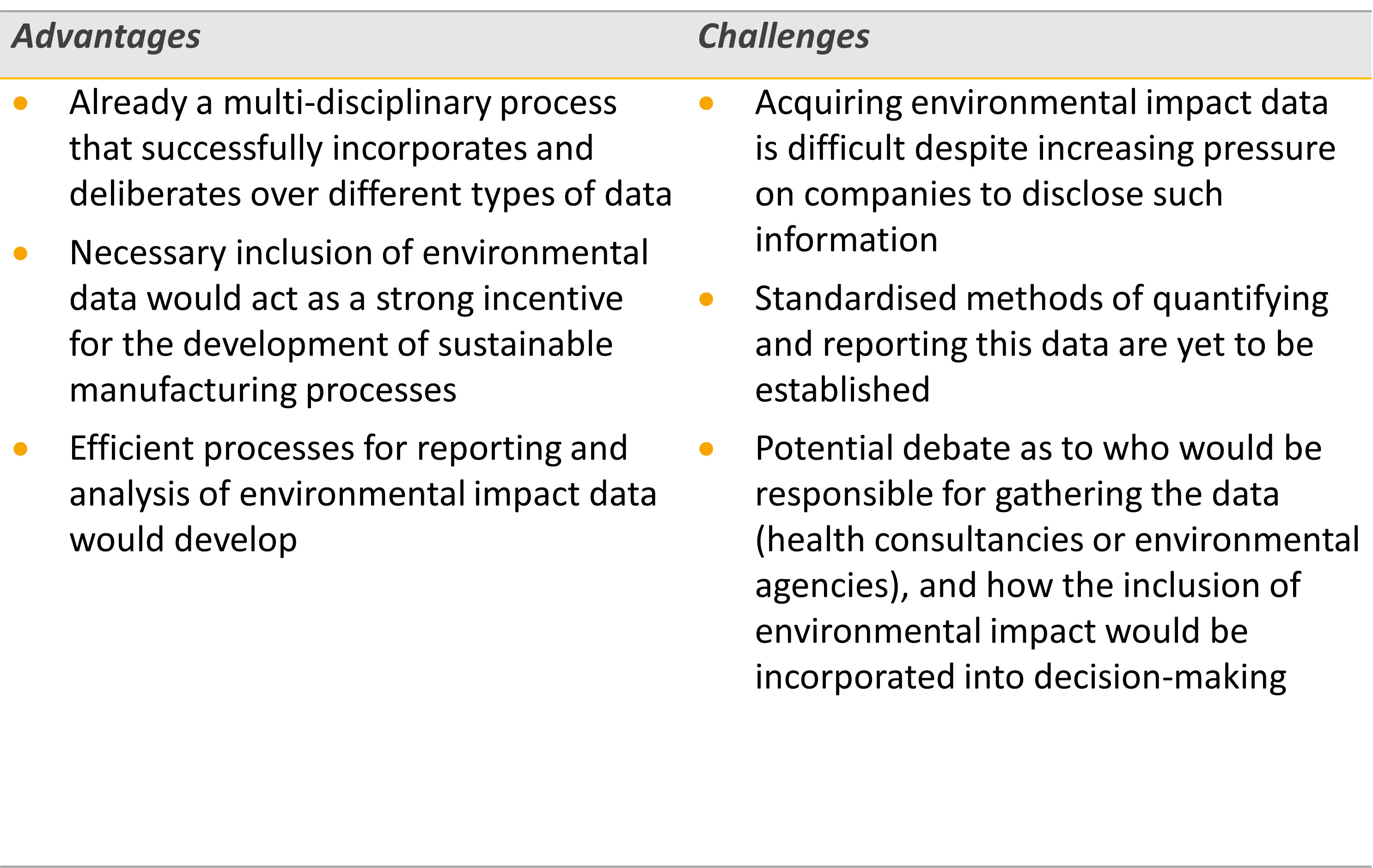
There are specific pros and cons associated with each of the individual approaches described above. However, there are perhaps advantages and challenges common to all methods and these are described in Table 1.
Table 1: Advantages and challenges of integrating environmental impact data into the HTA process
Research suggests that HTA agencies have started to collect and use environmental impact data; however, it is not happening routinely. In one publication, the authors report that out of 14 HTAs from six countries – Pharmaceutical Benefits Advisory Committee (PBAC) in Australia, Canada’s Drug Agency (CDA), Haute Autorité de Santé (HAS) in France, Sistema Sanitario Regione Liguria (ALISA) and Programma Regionale HA Dispositivi Medici (PHRDM) in Italy, Institute for Clinical and Economic Review (ICER) in the US, and National Institute for Health and Care Excellence (NICE) in the UK – environmental factors seemed to have an impact on the final decision in six HTAs but was not the main decision driver in any. Notably, seven HTA agencies in Canada, France, UK, USA, Netherlands, Sweden, Spain have produced policies or methodology guidelines that include environmental considerations (9).
Key takeaways
There are several key takeaways to note:
- If healthcare is to play its part in reaching the net zero targets set by governments, change in thought and method of procurement of health technologies is required.
- Single organisations will not be able to overcome the challenges alone and it will take a collaborative effort between pharmaceutical companies, healthcare providers, governments, regulatory authorities, and patients.
- While there are challenges associated with any change in process, the way the current HTA process has evolved overtime suggests that it is possible to integrate environmental data.
- It is encouraging to see that several HTA agencies have started exploring how this integration could take place.
If you would like to learn more about HTA submissions (including systematic reviews, health economic modelling, and medical writing), please contact us at Source Health Economics, a HEOR consultancy specialising in evidence generation, health economics, and communication.
References
- IPCC. Available at: https://www.ipcc.ch/assessment-report/ar5/ (last accessed 23rd October 2024). 2014
- Pichler P-P. Environ. Res. Lett. 2019;14(6)
- NHS England. Available at: https://www.england.nhs.uk/greenernhs/ (last accessed 23rd October 2024).
- NHS England Carbon Emissions Carbon Footprinting Report. September 2008 (updated August 2009)
- UNFCC. Available at: https://unfccc.int/ (last accessed 23rd October 2024)
- Greenwood Dufour B. Int. J. Environ. Res. Public Health 2022, 19, 12017.
- Toolan M. International Journal of Technology Assessment in Health Care, 39(1), e13, 1–4
- McAlister S. Lancet Planet Health 2022; 6: e993-99
- Szawara P. Poster by IQVIA presented at ISPOR Europe 2023
- Thomson D. Cochrane Database of Systematic Reviews 2022, Issue 3. Art. No.: ED000156
- NICE guidance on Asthma inhalers and climate change. https://www.nice.org.uk/guidance/ng80/resources/inhalers-for-asthma-patient-decision-aid-pdf-6727144573 (last accessed on 23rd October 2024)