Written by Susan O’Connell and Sara Bartlome
Introduction
Systematic literature reviews (SLRs) seek to answer a specific research question by translating the question into search terms and developing a literature search strategy to effectively identify relevant published evidence. In health economics and outcomes research (HEOR), SLRs are essential for identifying clinical and/or economic evidence gaps and informing new research. As such, SLRs in HEOR also support the development of materials designed to communicate product value and play an instrumental role in reimbursement decision-making for new medical interventions and technologies. Accurately structuring SLR questions to target the most relevant evidence for inclusion is fundamental in shaping evidence-based healthcare recommendations.
What is the PICO?
Getting the best from an SLR first requires a clear and focused review question, which serves as the foundation for developing a strong literature search. Translating this question into an accurate search strategy is therefore a critical step and is best achieved by applying a suitable framework. Different frameworks are selected for a de novo SLR based on the type of research question, common frameworks include:
-
- PICO (Population, Intervention, Comparator, Outcomes)
- PICOS (Population, Intervention, Comparator, Outcomes, Study type)
- SPICE (Setting, Perspective, Intervention, Control, Evaluation)
- SPiDER (Sample, Phenomenon of interest, Design, Evaluation, Research type)
The PICO is one of the most widely recognised approaches to framing review questions for clinical SLRs, and having a well-defined PICO increases the robustness and applicability of the SLR findings. Going forward, PICO surveys and prioritisation are a key step in the new European Union (EU) Joint Clinical Assessment (JCA) process, aiming to ensure that the final JCA output is relevant to all EU Member States.
Why is framing the SLR question so important?
Translating a review question into a well-defined PICO means that accurate search strategies can be developed to identify relevant published evidence. Well-defined PICOs can support appraisal of the quality of the evidence, which in turn can strengthen clinical and/or cost effectiveness cases for medical interventions and technologies. Conversely, a poorly defined PICO will limit the applicability and usefulness of an SLR.
In addition, an SLR is often not developed in isolation. Instead, they are used to support the development of other materials, such as global value dossiers (GVD), health technology assessments (HTA), licensing submissions, patient and product information documents, and are included as key evidence to support clinical decision-making. Therefore, a well-designed PICO framework can be a powerful tool for market access.
Key considerations for maximising a PICO
A single PICO framework may not answer all aspects of a research question, therefore spending time carefully exploring the key objectives of the questions to be answered can significantly impact the relevance of the search strategy. This considered approach to developing the framework helps to refine PICO definitions and provides an indication of whether multiple PICOs may be needed to ensure a comprehensive and targeted search strategy (Table 1).
Table 1: Key considerations when developing a new PICO framework for HEOR 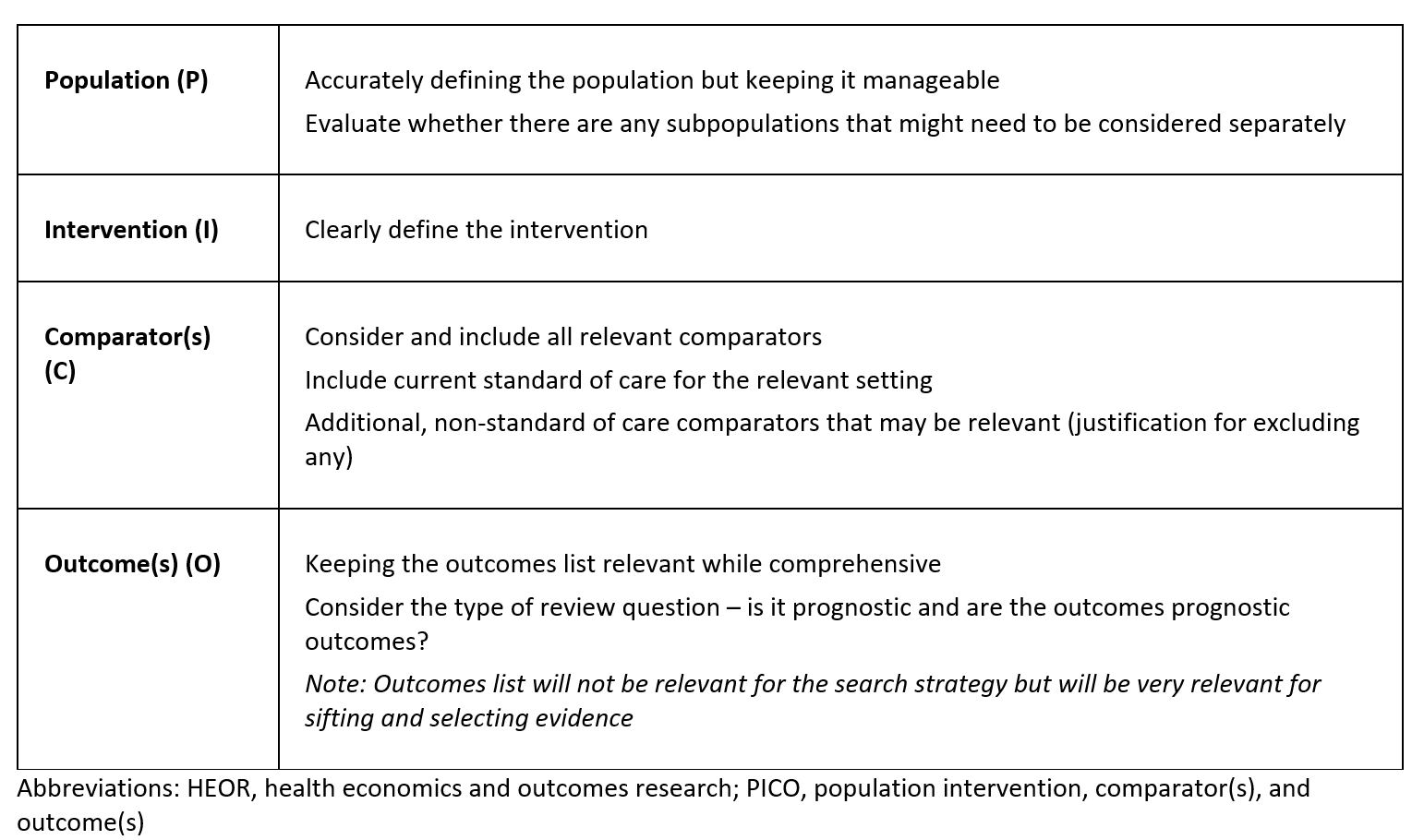
Even for seemingly straightforward review questions, there are many underlying factors to consider before building search strategies. As such, it is crucial to refine the PICO before conducting searches to avoid discovering later that the question was too broad, insufficiently specific, or did not effectively address the intended objective. Furthermore, maximising the PICO early in the process creates a structured and objective approach to answering the research question, ensuring that the framework guides the evidence search, rather than allowing existing evidence to dictate PICO development.
Including key stakeholders such as patient representatives or clinicians is an important part of the PICO development process. Therapy area experts can offer insight into the nuance of a disease that may not be immediately obvious, while patient representatives are crucial, particularly when there is a need to prioritise outcomes. As well as therapy area experts informing PICO development, including review methodologists (information specialists, systematic reviewers) ensures that PICOs are kept focused, guiding stakeholders and ensuring that there is a clear understanding of what the PICO can deliver. The importance of diverse input during the development process has newly been brought to light with the advent of the EU JCA. Stakeholder insights on a variety of EU Member States and healthcare frameworks will become increasingly relevant for PICO development in JCA, shaping companies’ subsequent strategic market access decisions.
Conclusion
The PICO forms the backbone of the review, providing a supportive structure to systematic analyses. Maximising the PICO not only strengthens the review but also enhances the impact and usefulness of its conclusions. However, if the PICO is poorly defined, significant time and effort may be wasted generating a systematic review that does not achieve its intended aims.
If you would like to learn more about PICO scoping and development, please contact Source Health Economics, an HEOR consultancy specialising in evidence generation, health economics, and communication.