Written by Kiera Lander, Assistant Project Manager
The National Institute for Health and Care Excellence (NICE) has long been at the forefront of health technology assessment (HTA), ensuring that patients have access to innovative, efficacious medicines. Recently, NICE has piloted and implemented a more flexible approach to HTA known as the proportionate approach to technology appraisals (PATT), which aims to streamline the assessment process for simpler, low-risk treatments.
In this blog, we will discuss key features of PATT and briefly summarise the pilot studies conducted to date.
The rationale for a new approach
The proportionate approach aims to provide an efficient and flexible framework for evaluating healthcare technologies, and strike a balance between providing access to innovative treatments, affordability, and timely recommendations. This approach recognises that not all treatments require the same level of scrutiny and that simpler submissions can benefit from a light touch evaluation process. The use of a proportionate approach for low-risk treatments seeks to increase appraisal capacity by 20%, as the time saved across streamlined appraisals will be dedicated to new evaluations and those requiring a more complex appraisal process.
In an introductory video conference on PATT, NICE discussed how it may benefit key stakeholders, including patients and the NHS, life sciences, and NICE, as summarised in Figure 1.
Figure 1: Potential benefits to stakeholders
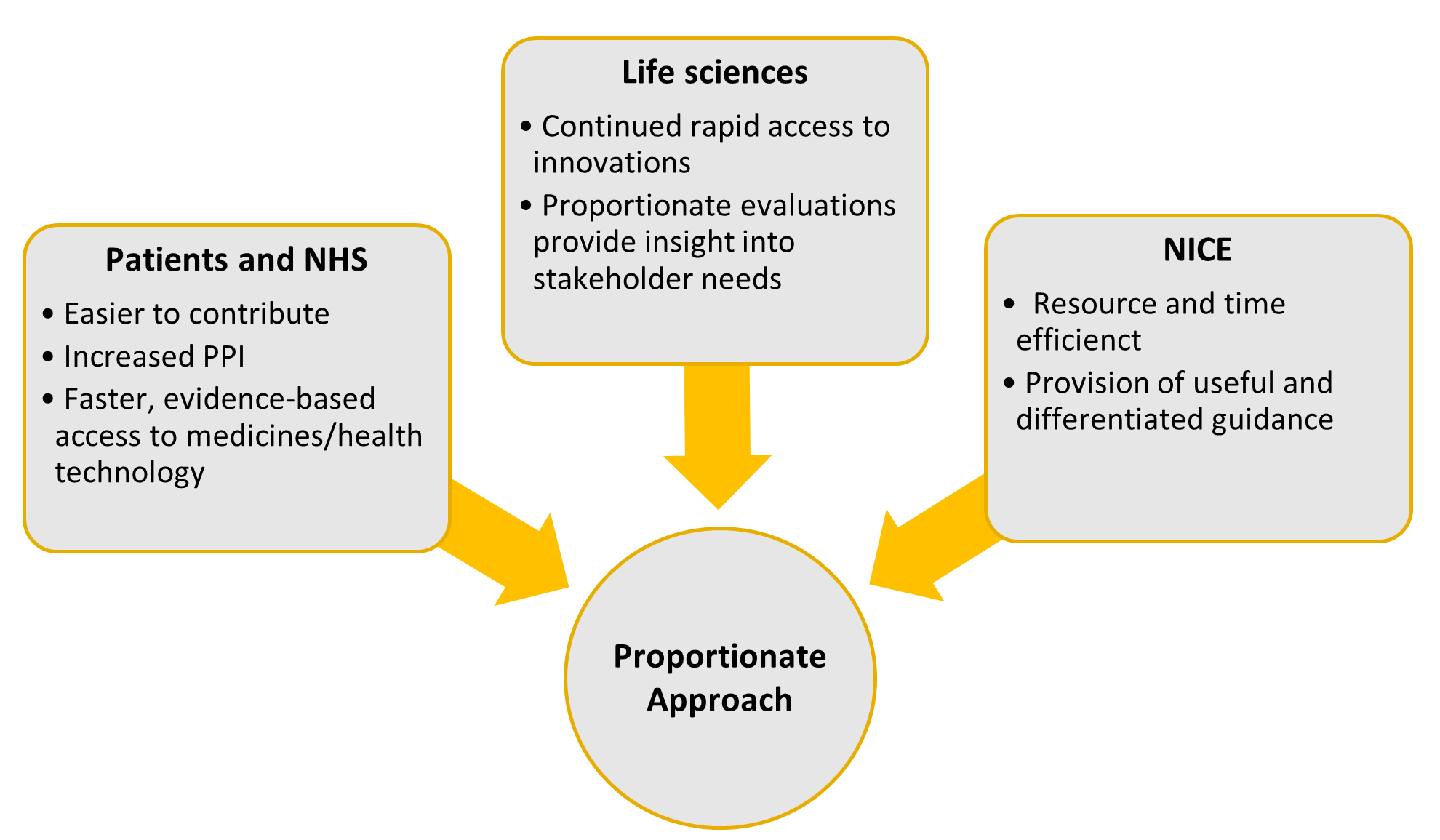
Abbreviations: NICE, National Institute of Health and Care Excellence; NHS, National Health Service; PPI, public and patient involvement.
A ‘phased introduction’
There are two phases to the introduction of PATT. In phase one, two new methods were introduced: a cost-comparison process and a streamlined decision making process. Phase one involved a test-and-learn approach in which six submissions were piloted with real-time consultations with stakeholders and industry partners (Table 1).
Phase two of PATT is ongoing, and involves introducing the Pathway Approach, emphasising ‘high value steps’, and exploring Rapid Entry to Managed Access (REMA).
- Pathway Approach: Aims to address the increasing number of treatments in certain disease areas. It involves the evaluation of multiple treatments for one patient population in a singular economic model and uses real-world evidence to reflect NHS practices. The Pathway Approach is currently being evaluated in two additional pilot programmes in renal cell carcinoma and non-small-cell lung cancer.
- High Value Steps: This involves iteratively improving ‘ways of working’ by optimising and innovating committee meeting management, implementing additional internal efficiencies, and assessing proportionate reviews.
- REMA Initiative: In collaboration with the NHS, the REMA process aims to expedite patient access to new technologies via the Cancer Drugs Fund.
What is PATT and how does it differ from the existing process?
Since the first phase of PATT (2022), process changes have been published in NICE’s interim methods and process guide, which include changes in scoping, scheduling of topics, handling of confidential information, the new cost-comparison process, topic progression after evidence critique, an opt-in system for technical engagement, and committee decisions outside of formal meetings.
Table 1: PATT processes
| The new cost-comparison process | Streamlined decision-making | |
| Features |
|
|
| Results from pilot studies |
|
|
| Pilot technology appraisals |
Summary of the pilot studies and their outcomes
Six therapies have been recommended via cost comparisons and streamlined decision-making as part of the pilot studies. The pilots demonstrated faster access to treatments, with assessments being completed 7–20 weeks quicker than standard processes and a 17% increase in NICE’s capacity. Table 2 outlines the pilot studies conducted using new PATT processes, which benefitted approximately 175,000 patients.
Table 2: First phase pilot studies
| Treatment | Indication | Weeks faster than standard process |
| Bimekizumab | Psoriatic Arthritis | 12 |
| Somatagron | Growth Disturbance | 7 |
| Nintedanib | Idiopathic Pulmonary Fibrosis | 8 |
| Vutrisiran | Amyloidosis | 20 |
| Eptinezumab | Migraine | 8 |
| Nivolumab | Resectable Non-Small-Cell Lung Cancer | 9 |
The next phase of PATT will include a report (expected in Q2 2024) summarising insights from the pilot appraisals of the Pathway Approach.
NICE’s proportionate approach represents a significant step forward in optimising technology appraisals. By tailoring the evaluation process to the complexity and risk profile of treatments, NICE is paving the way for more timely and informed decision-making in healthcare.
The next blog will explore the limitations of PATT, and a more in-depth evaluation of NICE’s aims in relation to the pilot studies.
- For further information on the proportionate approach, see NICE’s interim methods and process guide and the 2022/23 final report
- If you would like to discuss the implications of PATT and your plans for future HTA submissions, please get in touch with us at Source Health Economics




